42 legal requirements for dispensing labels uk
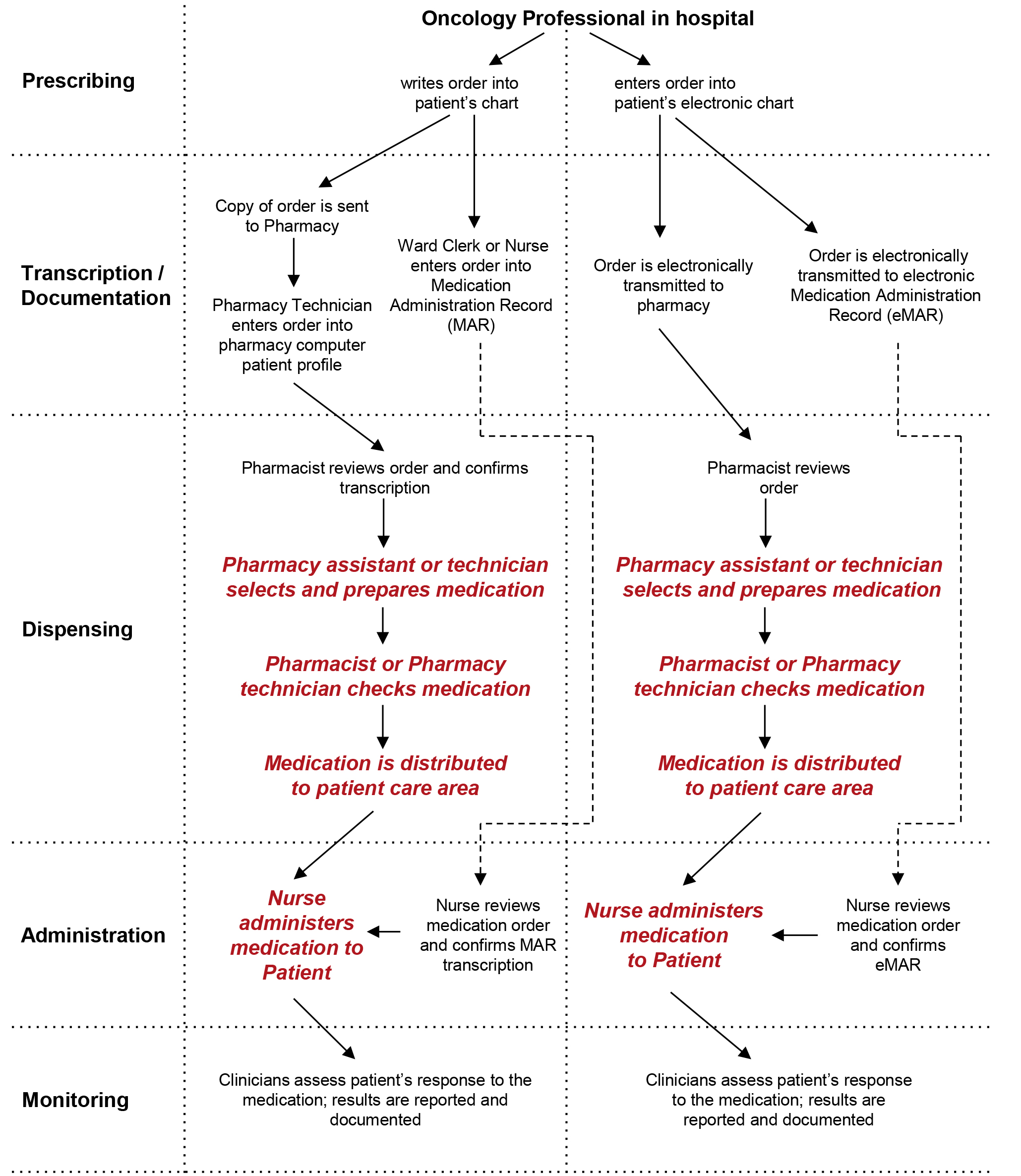
Dispensing a prescription - PSNC Website EPS Dispensing. EPS Submission. EPS Tokens. EPS Prescription Tracker. eRD. Medicines Database (dm+d) RTEC Exemption Checking. Records, Data Security & IG. Back to Records, Data Security & IG. Medicines: packaging, labelling and patient information leaflets Labels must be clear. Healthcare professionals and patients must easily be able to identify the medicine by the label. You should use the letters CD in an inverted triangle if your product is a...
Alcohol Labelling Requirements Explained | Premier Labels As the Food Standards Agency explains, any type of alcoholic beverage needs to be marked with alcohol strength where it is above 1.2%. Where the volume content is above 1.2%, it needs to be labelled with the strength to a maximum of one decimal place in a 'X% vol' format (with X being the strength). Alternatively, brands might choose to ...

Legal requirements for dispensing labels uk
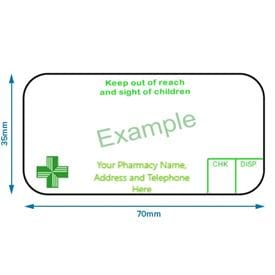
Guidance for cautionary and advisory labels | About | BNF | NICE Although not listed in the BNF these labels should continue to be used when appropriate; indeed, 'For external use only' is a legal requirement on external liquid preparations, while 'Keep out of the reach of children' is a legal requirement on all dispensed medicines. Product labelling: the law - GOV.UK If you're a retailer, you must display: the price of products - this must be in sterling (pounds and pence) and include VAT where applicable the price of a single item (the 'unit price') for... United Kingdom - Labeling/Marking Requirements UKCA markings must only be placed on a product by the manufacturer or an authorized representative. When affixing the UKCA marking, the manufacturer takes full responsibility for the product's conformity with the requirements of the relevant legislation. The UKCA marking must only be used to demonstrate conformity with the relevant UK legislation.
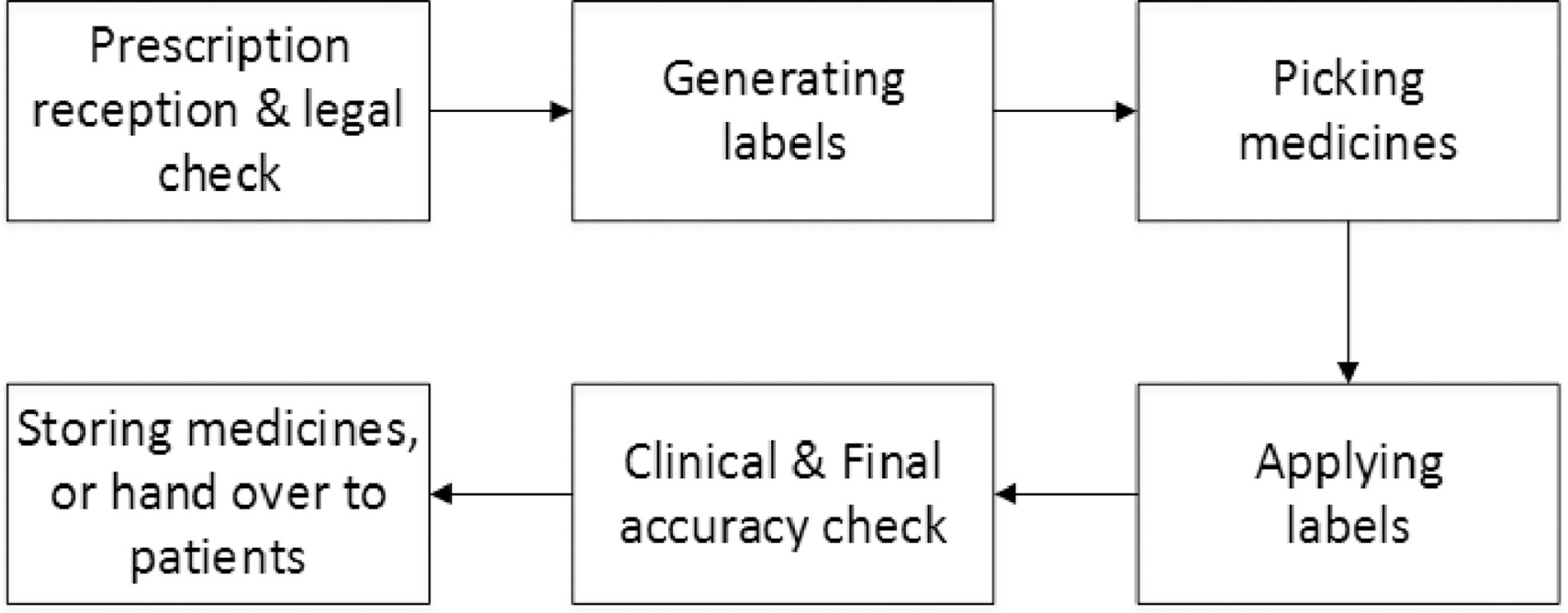
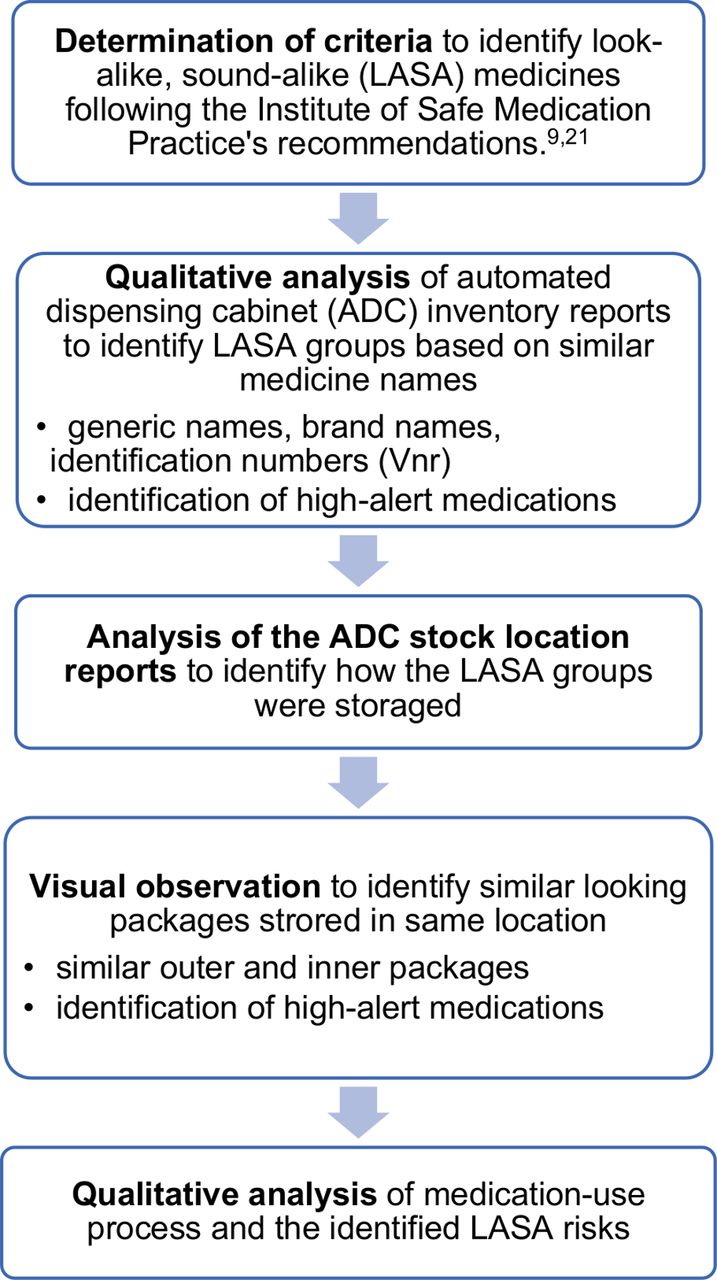
Legal requirements for dispensing labels uk. Candle Label Requirements - EU Edition • Armatage Candle Company You would list 186 grams on your label. This means you don't take the finished candle and set it on a scale - you have to know how much you're pouring into the jar. You can approximate this weight, especially if you're pouring in batches. 4. Product name or identifier Article 18 specifies you need to include two items: Rules for the sale, supply and administration of medicines for ... - GOV.UK The medicines must be: sold/ supplied by registered chiropodists/podiatrists pre-packed sold/ supplied during professional practice by those with a certificate of competence in the use of the... Optimising Dispensing Labels and Medicines Use The Human Medicines Regulations 2012 introduce changes to labelling and medicines-use which advance the clinical role of pharmacists in supporting people to get the most from prescribed medicines across the UK, providing greater clinical flexibility for prescription intervention. Dispensing Medicines - PSNC Website Pharmacies are required to maintain a record of all medicines dispensed, and also keep records of any interventions made which they judge to be significant. The Electronic Prescription Service (EPS) is also being implemented as part of the dispensing service. Service Specification
Legal requirements for food labels | nibusinessinfo.co.uk Legal requirements for food labels. Guide. For most prepacked foods, you must provide on their label the following details: the name of the food. an ingredients list. information on certain foods causing allergies or intolerances that were used in the manufacture or preparation of a food. the quantity of certain ingredients. PDF Amendments to the Human Medicines Regulations 2012: 'hub and spoke ... • Clarify the dispensing label requirements of the Human Medicines Regulations 2012, in particular by updating the labelling requirements for monitored dosage systems to reflect current practice and by ensuring products supplied under patient group directions have a dispensing label in line with professional guidance; and Are Care Labels a Legal Requirement? - Business Services Week UK For such a small label, there are four areas of information that are required to be displayed: Fibre Content Mandatory for care labels in the UK, the fibre content of textiles must include information on the main fibre types used along with the percentages. The information must be easily understandable to the consumer, eg 80% wool, 20% cotton. PDF BPGLPM tracked for EoT 041120 legal cleared - GOV.UK ,w pd\ eh qhfhvvdu\ lq vrph fdvhv wr h[suhvv wkh vwuhqjwk dv txdqwlw\ shu xqlw yroxph dqg dovr dv wkh wrwdo txdqwlw\ shu wrwdo yroxph 5hihuhqfh wr wkh wrwdo
Labels and the Law (UK & EU) In the EU and the UK, manufacturers are legally required to state what textiles a garment is made out of. You must give an exact percentage of any material that comprises more than 15% of the total weight of the product, and every material must be listed on the label. Expressions like "100% cotton" or "pure wool" are legally-protected ... Labelling of Spirits in the UK: requirements in relation to the ... Labelling of Spirits (UK) What are the legal requirements for labelling alcoholic spirit drinks within the UK? The legal requirements for the labelling of spirit drinks produced and sold within the UK are found in the Spirit Drinks Regulations 2008, which enforces the European Directive (EC) 110/2008 into UK law.These laws protect consumers against fraudulently labelled products. Packaging & Labelling Requirements Guide | Handy Labels Requirements on the Primary Packaging On the primary packaging for cosmetics you will need to include: Contact details of responsible person or organisation Ingredient listings Batch code or number Shelf life Some means of identifying the function The average net weight or number of the product (s) Best before date Guidance on Prescribing, Dispensing, Supplying and Administration of ... Guidance on Prescribing, Dispensing, Supplying and Administration of Medicines Some of our publications are also available in hard copy, but this may entail a small charge. For more information and to order a hard copy please call 0345 772 6100 and select option five. The line is open Monday-Friday (excluding bank holidays) between 10am-4pm.
Pharmacy dispensing models and displaying prices on medicines ... - GOV.UK The policy proposal on MDS and in addition to the minimum requirements around information on the dispensing label would be to enable prescribers and pharmacists to include a description of...
The Human Medicines Regulations 2012 - Legislation.gov.uk Sale and supply of starting materials. 33. Offence concerning data for advanced therapy medicinal products. 34. Offences: breach of regulations and false information and defence concerning starting materials. 35. Penalties. Conditions for holding a manufacturer's licence. 36.
Packaging and labelling | Food Standards Agency Labelling requirements. For non-prepacked food, the name of the food, presence of any of the 14 allergens, and a QUID declaration (for products containing meat), must be provided to consumers. This can be done: (a) on a label attached to the food, or. (b) on a notice, ticket or label that is readily discernible by an intending purchaser at the ...
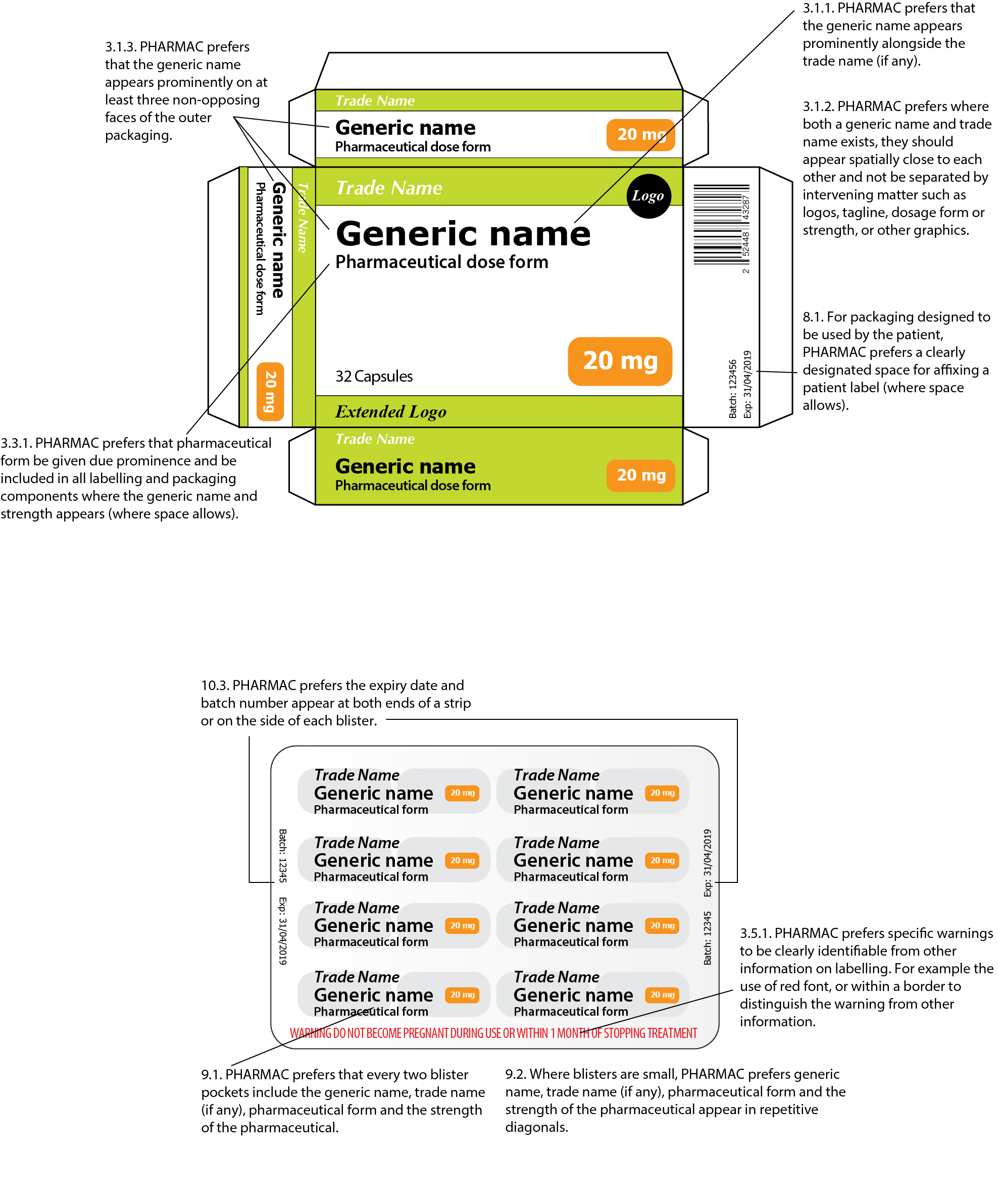
Best practice in the labelling and packaging of medicines Guidance Best practice in the labelling and packaging of medicines This guidance explains the legal framework for labelling and packaging as described in UK legislation and gives best...
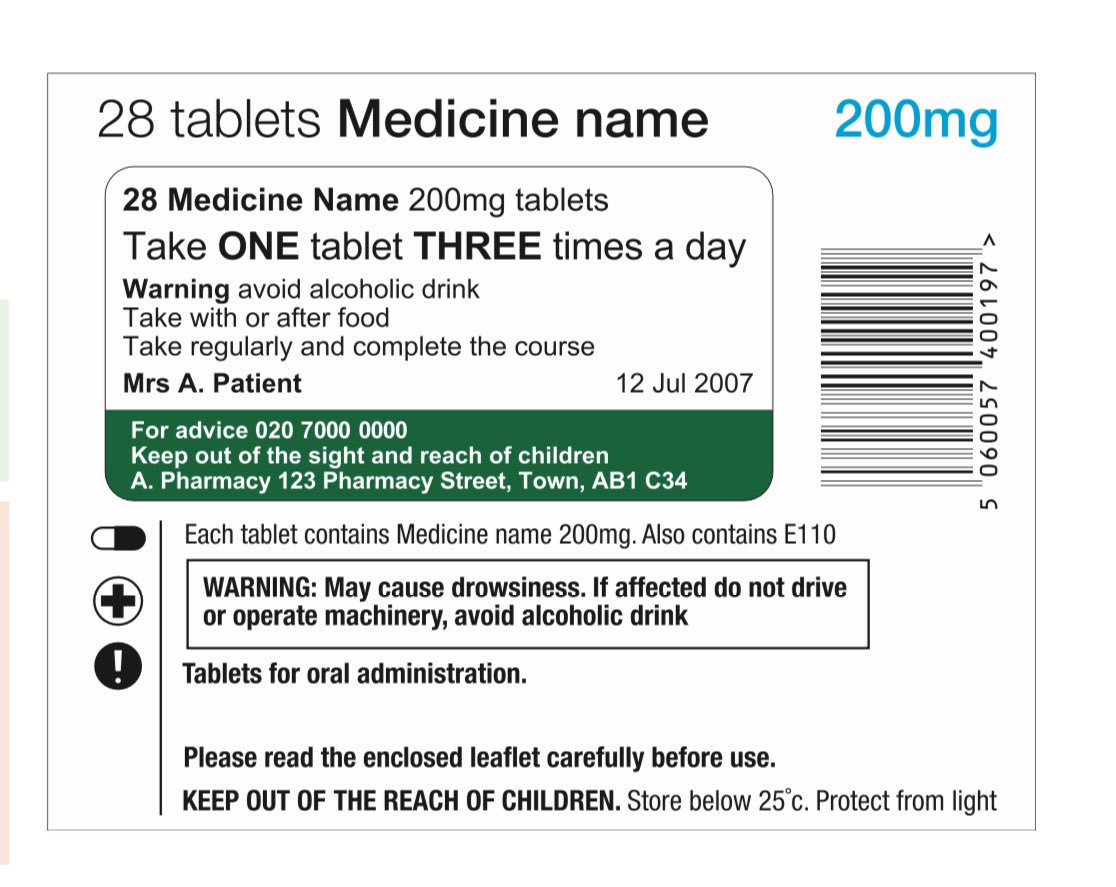
Prescription writing | Medicines guidance | BNF | NICE The age and the date of birth of the patient should preferably be stated, and it is a legal requirement in the case of prescription-only medicines to state the age for children under 12 years. These recommendations are acceptable for prescription-only medicines. Prescriptions for controlled drugs have additional legal requirements.
The Human Medicines Regulations 2012 - Legislation.gov.uk 217. — (1) For the purposes of this Chapter, a prescription only medicine is not sold or supplied in accordance with a prescription given by an appropriate practitioner unless the following conditions are met. (2) Condition A is that the prescription is signed in ink by the appropriate practitioner giving it. (b) in the case of a health ...
FDA Issues New RX Label Requirements | RX Label Requirements for Opioids On July 1, 2019, the Food and Drug Administration issued new prescription label requirements . Medication that will be included under these new rules include those that are categorized under the Controlled Substances Act. Controlled substances are highly potent and potentially addictive medications, including opioids.
The Medicines (Labelling) Amendment Regulations 1992 - Legislation.gov.uk Special requirements for the labelling of the name of medicinal products for human use 4D. — (1) In any case where— (a) a relevant medicinal product is available in more than one pharmaceutical...
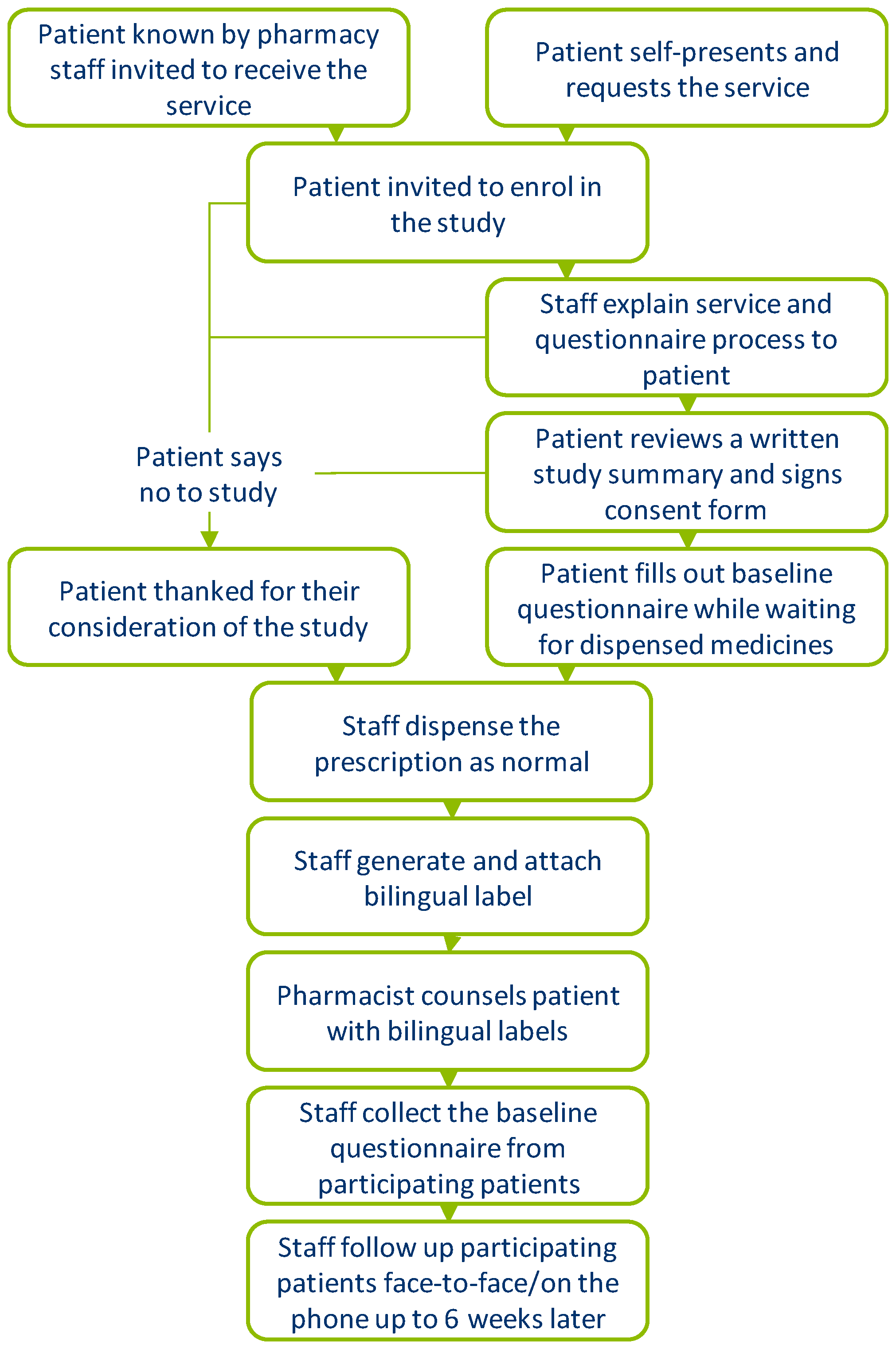
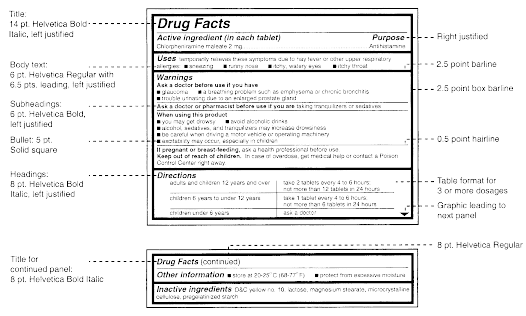
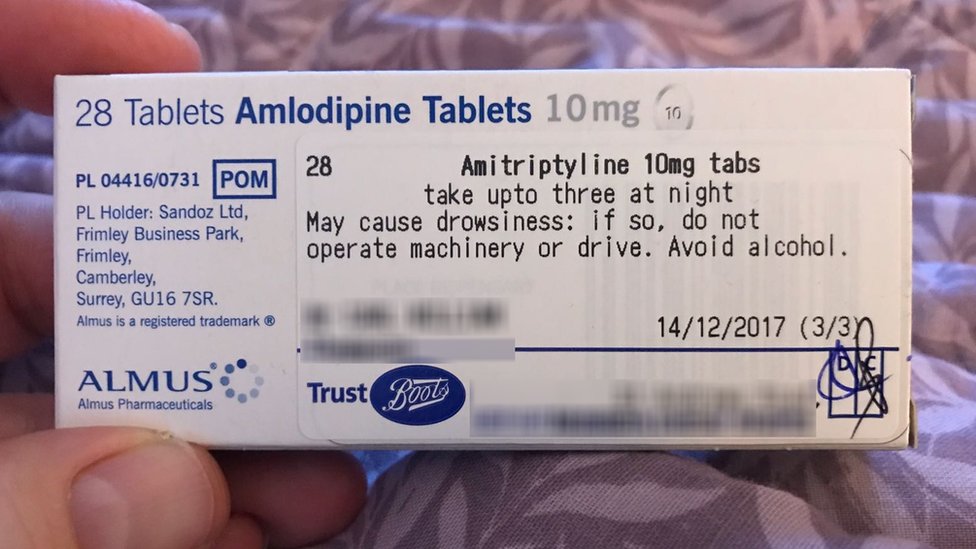
Labelling standards - Pharmacy Forum UK "apply 1-2 times a day" (bad practice to put numbers on labels also somebody with bad eyesight could see 12) "take two four to six hourly" (quite a few patients probably dont understand this) "take 1 3 times/day" "take ONE cap three times a day (ADVICE) after food" (use proper english!!!!!) "take two morning and night"
Legal & Optional Requirements for Labelling - Stephens Scown Mandatory requirements Bottles & Cans The following information must be provided directly on the packaging or on an attached label on both bottles and cans: 1. The legal name of food For example, "beer". The name should be in the language (s) relevant to the countries in which the product is to be marketed.
United Kingdom - Labeling/Marking Requirements UKCA markings must only be placed on a product by the manufacturer or an authorized representative. When affixing the UKCA marking, the manufacturer takes full responsibility for the product's conformity with the requirements of the relevant legislation. The UKCA marking must only be used to demonstrate conformity with the relevant UK legislation.
Product labelling: the law - GOV.UK If you're a retailer, you must display: the price of products - this must be in sterling (pounds and pence) and include VAT where applicable the price of a single item (the 'unit price') for...
Guidance for cautionary and advisory labels | About | BNF | NICE Although not listed in the BNF these labels should continue to be used when appropriate; indeed, 'For external use only' is a legal requirement on external liquid preparations, while 'Keep out of the reach of children' is a legal requirement on all dispensed medicines.
























Post a Comment for "42 legal requirements for dispensing labels uk"